Many companies think obtaining a CE-EMC certificate is the finish line. In reality, it's the starting point for dynamic compliance management. The essence of EU regulations isn't a piece of paper but the manufacturer's ability to prove ongoing compliance with current requirements at any time. In 2025, with unprecedented strengthening of EU Market Surveillance, understanding the true logic behind "documentation" and "validity" is directly tied to your product's stable sales in Europe, avoiding recalls, detentions, and hefty fines.
Per the EU EMC Directive (2014/30/EU), the manufacturer must create a Technical File – the core evidence proving compliance. This isn't just application paperwork but must be kept by the manufacturer (or its EU Authorized Representative) for at least 10 years, ready for inspection by EU surveillance authorities.
Below is the standard 2025 Technical File checklist, a linked "chain of evidence":
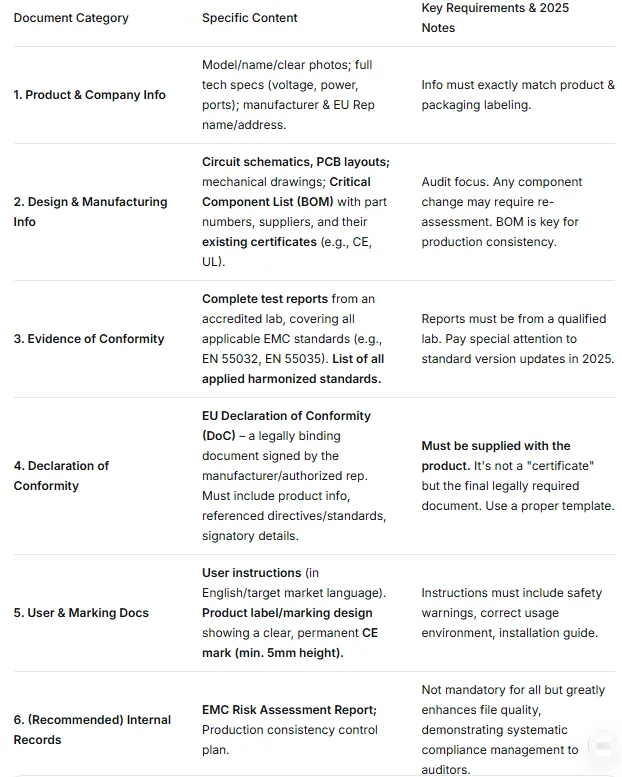
Core Reminder: For products with wireless functions (Wi-Fi, Bluetooth), meeting the EMC Directive alone is insufficient. They must also comply with the Radio Equipment Directive (RED), requiring additional RF test reports (e.g., EN 300 328, EN 301 893) in the Technical File.
II. Validity Period: The Biggest Misconception & Dynamic Management
This is the most misunderstood area. Remember a core principle: The CE certification itself (embodied by the DoC) has no official fixed expiry date. Its "validity" is dynamic, lasting until one of these conditions occurs:
1.Three Triggers for Re-assessment (The Real Reasons a Certificate "Expires"):
-Regulation/Standard Update: The most common cause. EU harmonized standards are revised every 3-5 years. New versions enter a transition period (usually 6-24 months). After this, DoCs based on the old standard become invalid. (E.g., 2025 RED updates for 5G NR require certificate upgrades).
-Product Change Itself: Any modification affecting EMC characteristics (circuit design, key component replacement, major software upgrade, production process change) requires re-assessment of the Technical File and potentially re-testing.
-Certification Body Policy: While not mandated by law, many Notified Bodies set a 3-5 year validity for their "voluntary certificates." Also, platforms like Amazon often require CE test reports issued within 1-2 years, or they may request an update.
2.Management Differences by Risk Level:
-Ordinary Electronics: Typically use the "Self-Declaration" path. The DoC remains valid as long as the product and standards are unchanged. Industry practice suggests a systematic review every 5 years, especially during active standard update periods.
-High-Risk Products: For devices requiring Notified Body certification (e.g., medical devices under MDR), certificate validity is often tied to annual surveillance audits. Failure leads to suspension/revocation.
III. From Theory to Practice: A 2025 Compliance Roadmap
To ensure ongoing CE-EMC compliance, follow this 4-step dynamic management approach:
1.Establishment Phase: Create a "Living" Technical Archive: Build a complete, standardized Technical File library using the checklist above from the first certification. Treat it as a dynamic digital archive, not a static paper pile.
2.Monitoring Phase: Set Up Regulatory Alerts: Subscribe to update services from professional bodies or regularly check the EU Official Journal. Focus on the EMC Directive and core standards like EN 55032, EN 55035.
3.Change Control Phase: Enforce Strict Change Management: Implement an internal process where any product change (even a capacitor) must be assessed by an engineer for potential EMC impact. "Assess first, change later" is the rule. Consult your certification body if unsure.
4.Action Phase: Plan Proactively & Update: Upon confirming a standard update or major product change, immediately plan for re-testing or differential testing. Use the standard transition period to plan ahead, avoiding supply chain disruption from a "suddenly invalid" certificate. For high-risk products, ensure timely annual surveillance audits.
In essence, CE-EMC certification requires building and maintaining a system for self-demonstrating ongoing product compliance. The Technical File is the evidence repository, and dynamic "validity" management is the operational core. In the increasingly strict 2025 trade environment, only by transforming compliance from a "project task" to "daily management" can you ensure your product's steady journey in the European market.
BLUEASIA Tech: +86 13534225140** provides professional certification consulting services.
Related News